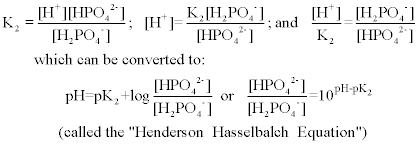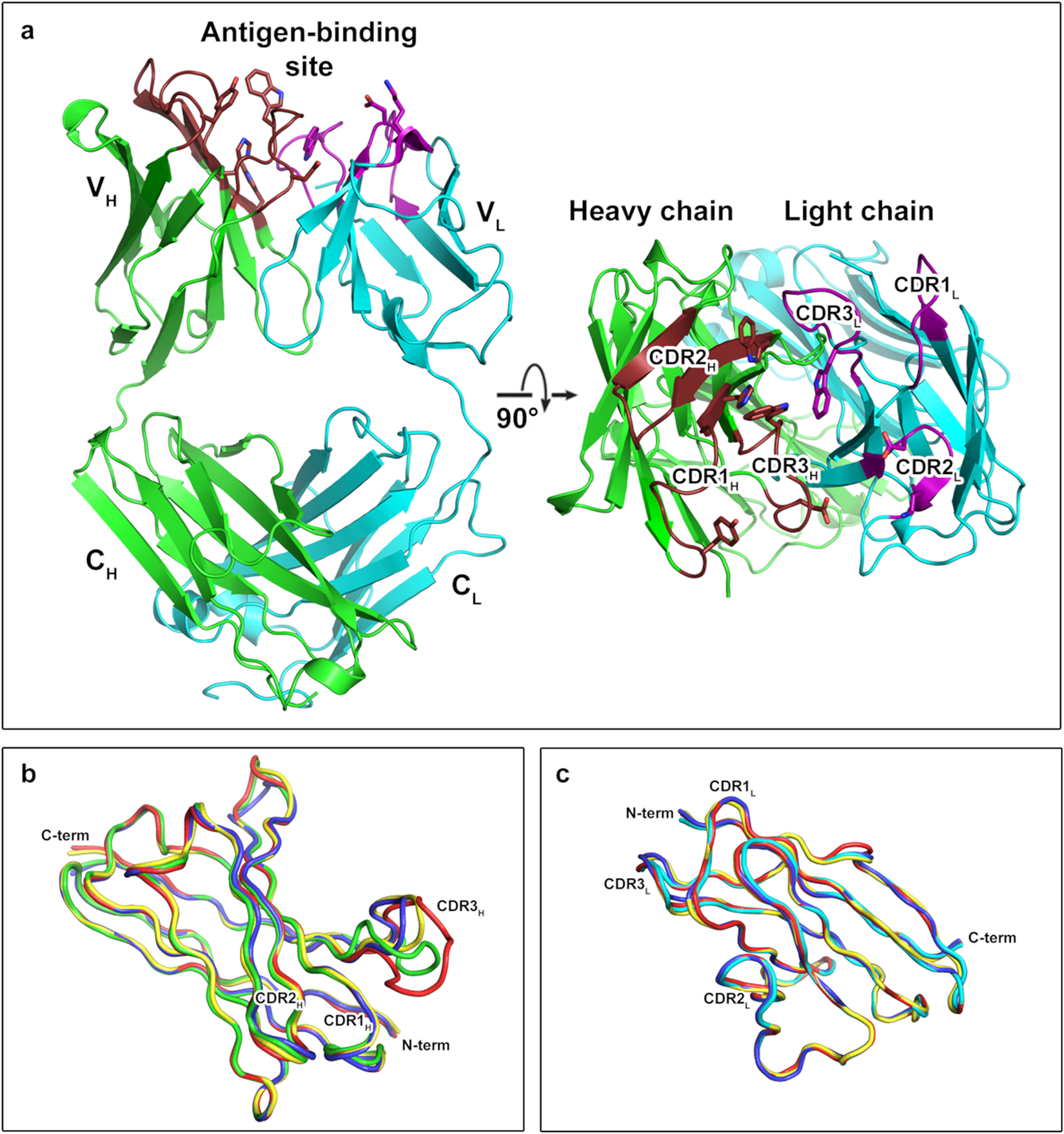
Structural insights into antigen recognition of an anti-β-(1,6)-β-(1,3)-D-glucan antibody | Scientific Reports

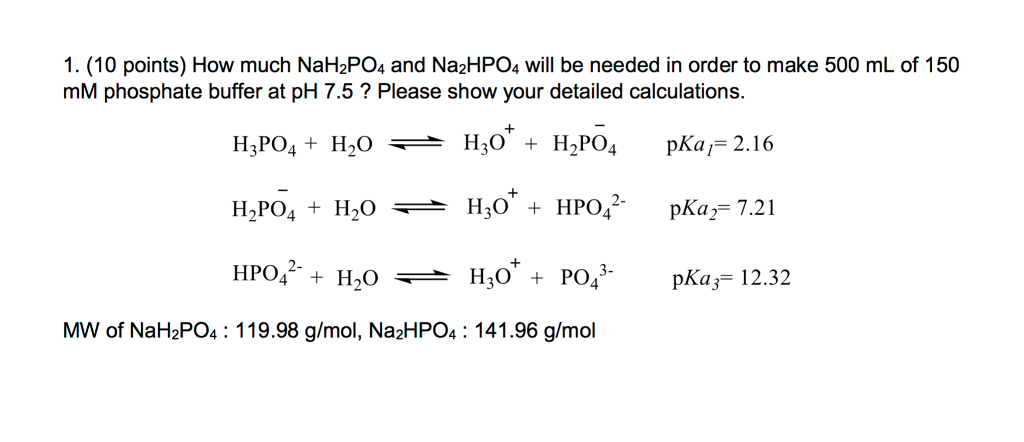
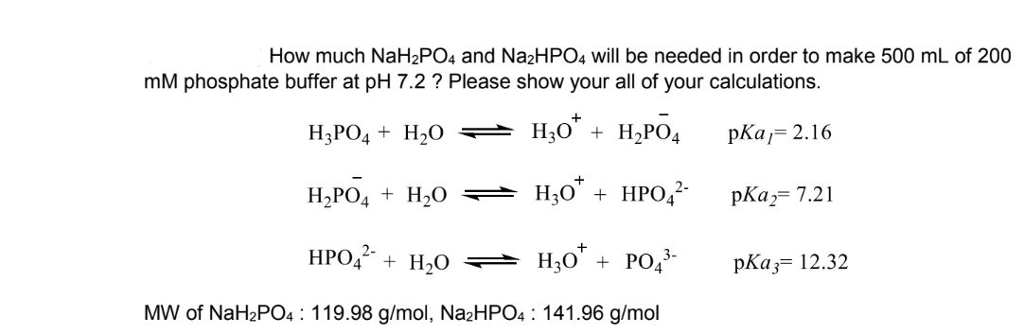
SOLVED: A phosphate buffer was made using the sodium salts of NaH2PO4 and Na2HPO4. The pH was recorded as 8.3 but you desire a pH of 7.5. To establish the desired pH,

Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions | Journal of Agricultural and Food Chemistry

Thermodynamic and Kinetic Analysis of Isothermal Titration Calorimetry Experiments by Using KinITC in AFFINImeter | SpringerLink