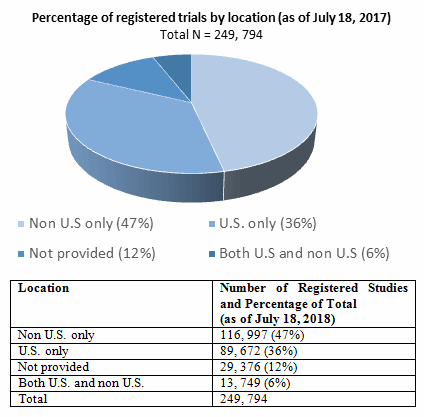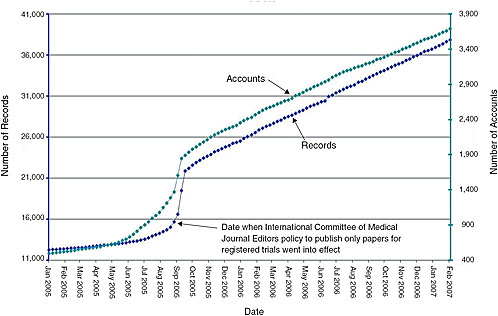
7 Enhancing the Value of Clinical Trial Registration | Challenges for the FDA: The Future of Drug Safety: Workshop Summary | The National Academies Press

Peer reviewed evaluation of registered end-points of randomised trials (the PRE-REPORT study): protocol for a stepped-wedge, cluster-randomised trial | BMJ Open

Regulatory Approval – Clinical Trial Medical Monitoring Plan | Online Clinical Research Courses In India
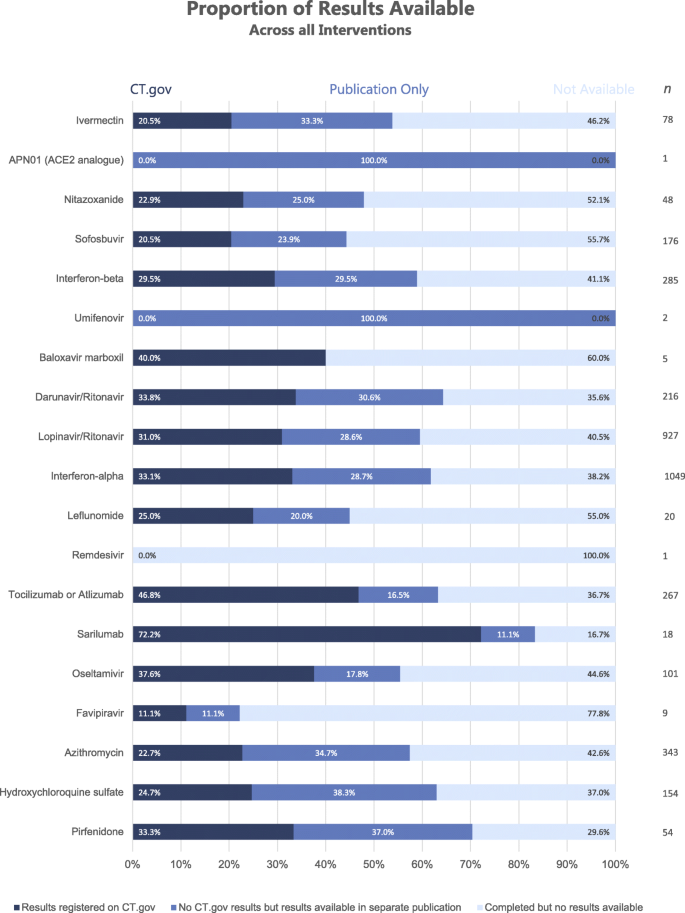
Missing clinical trial data: the evidence gap in primary data for potential COVID-19 drugs | Trials | Full Text

Overview of phase IV clinical trials for postmarket drug safety surveillance: a status report from the ClinicalTrials.gov registry | BMJ Open

Challenges for funders in monitoring compliance with policies on clinical trials registration and reporting: analysis of funding and registry data in the UK | BMJ Open